FDA Approves Gardasil 9: More is Better for Cervical Cancer Prevention
8/07/2015, Karen Honey, PhD
In early December, the U.S. Food and Drug Administration (FDA) announced the approval of a new vaccine—Gardasil 9—that prevents infection with five more cancer-causing subtypes of human papillomavirus (HPV) than the current HPV vaccines, Gardasil and Cervarix.
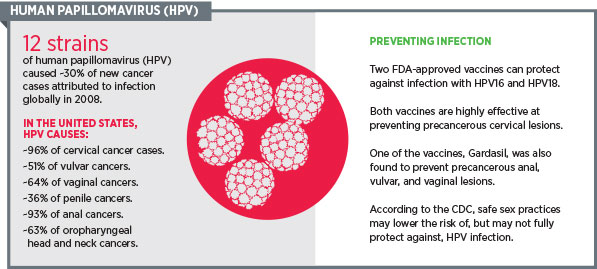
As discussed on this blog by my AACR colleague Srivani Ravoori, PhD, research shows that Gardasil 9 could prevent almost 90 percent of cervical cancer cases worldwide. It also has the potential to prevent more cases of vulvar, vaginal, and anal cancers than Gardasil and Cervarix can.
The reason Gardasil 9 has the capability to prevent more cases of cancer than Gardasil and Cervarix is that while Gardasil and Cervarix prevent infection with the two main cancer-causing HPV subtypes, HPV16 and -18, Gardasil 9 protects against an additional five cancer-causing HPV subtypes: HPV31, -33, -45, -52 and -58. Gardasil and Gardasil 9 also prevent infection by two subtypes of HPV that cause genital warts, HPV6 and -11.
Gardasil 9 is approved for the prevention of cervical, vulvar, vaginal, and anal cancers caused by HPV16, -18, -31, -33, -45, -52, and -58. It is likely that Gardasil 9 could also prevent a significant number of cases of anal cancer and cancers of the oropharynx—the part of the throat just behind the mouth—because more than 50 percent of U.S. cases of these cancers are related to HPV infections. However, research is needed to show that this is indeed the case.
Unfortunately, Gardasil 9 will only live up to its promise if those individuals for whom it is recommended—females ages 9–26 and males ages 9–15—get vaccinated. With the latest data from the Centers for Disease Control and Prevention estimating that just 38 percent of adolescent girls (ages 13–17) and 14 percent of adolescent boys completed a course of Gardasil or Cervarix in 2013, it is clear that more needs to be done to develop and implement effective strategies.
A number of recommendations to increase HPV vaccine update were made in the most recent President’s Cancer Panel Report, “Accelerating HPV Vaccine Uptake: Urgency for Action to Prevent Cancer,” which was released in February 2014, and there is optimism that we can make progress.
“We can prevent a generation of young Americans from having to go through the same experience I did,” said Bob Margolis, a seven-year survivor of HPV-related cancer and advocate for HPV awareness and vaccination, in the AACR Cancer Progress Report 2014.
Learn more about Margolis’ story in this video.
Get more information or read the original article here.